Flexible Batteries Unfold Technical Possibilities
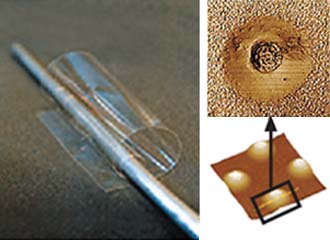 |
| Researchers sponsored by the Army Research Laboratory have built batteries using biological systems. The battery on the right is three one-thousands of a millimeter thick. |
This research is part of ongoing work sponsored by the U.S. Army Research Laboratory (ARL) and a team of scientists at the Massachusetts Institute of Technology (MIT). The researchers, led by materials science professor Angela Belcher, have developed a method to use viruses as nanoscale scaffolds to build batteries.
Belcher’s team is using a genetically modified M13 bacteriophage, a virus that only infects bacteria, to wire electrical materials to nanoscale ionically conducting materials. She explains that her group is focused on developing high-power batteries with rapid charge-discharge cycles that still can maintain adequate electrical capacity levels during repeated use.
The MIT researchers engineered the virus to “pick up” a single-walled carbon nanotube and to hold it. Describing the virus as a pencil, Belcher explains that on its writing end, it has a protein sequence in its DNA that adheres to the carbon nanotube. During this process, the virus also activates proteins that grow an iron phosphate substance, which forms the battery material.
Belcher’s team previously had used engineered viruses to build an anode by coating themselves with cobalt oxide and gold, then self-assembling to form a nanowire. In their current work, the scientists built a powerful cathode to pair with the anode. Iron phosphate was selected because it is an efficient conductor. The network of carbon nanotubes connected to the iron-phosphate-coated viruses creates a network of highly conductive material. The iron phosphate nanowires can be connected electrically to conducting carbon nanotube networks. Electrons can travel along these carbon nanotube networks, moving through the electrodes to the iron phosphate and rapidly transferring energy. The battery materials are synthesized in an inexpensive and nontoxic process that takes place at or below room temperature.
The prototype batteries are small coin cells identical to and interchangeable with common calculator batteries. The research team has used the experimental cells to power small electronics such as wristwatches and calculators. Belcher notes that it would not be very difficult to scale the battery up to power a laptop computer. However, she cautions that her team has not built any other batteries besides the coin cells. “We’re not going to try to scale anything until it’s a better material,” she says.
Materials remain a challenge because amorphous iron phosphate is not an ideal material for batteries. “It’s not ready to leave the lab. It will be ready to leave the lab when we have a material that gives us an even higher energy density,” Belcher says. The researchers now are focusing on replacing iron phosphate with other substances such as lithium manganese phosphate, lithium nickel phosphate and fluoride-based materials.
“It’s still at the applied basic science level because although we have the technique down in wiring electrically conductive materials to ionically conducting materials, we haven’t picked the best cathode material yet,” she says. Another consideration is that many of the cathodes with high energy densities have low kinetic capabilities, making them inferior conductors. Both high kinetic capability and high energy density are required for good battery materials, she shares.
The MIT team is working to improve substances that are less efficient electrical conductors than iron phosphate by linking them with nanoscale wiring to an efficient conductor. The researchers hope that this process will yield a structure with high conductivity. Belcher’s team also is attempting to attach multiple conductors running the length of the virus. She explains that the goal of this research is to determine if growing the ionically conducting material in the gap between the electrically conducing materials is a better way to transfer electrons.
 |
A small battery being tested at the Massachusetts Institute of Technology is shown providing power for a light-emitting diode. |
Potential applications for the technology include “pourable” batteries that take the shape of their container. For example, if a battery has to match a piece of equipment to avoid bulkiness, it could be poured directly into the device. Another area of work is on flexible and conformable batteries that can be woven into textiles. Researchers also are seeking to integrate the batteries into bulletproof materials to provide multifunctional systems that theoretically could power devices attached to ballistic vests, she says.
The viral technology also can be used in microbatteries, tiny power systems that can be attached to any type of surface. This capability will allow small devices such as laboratories on chips and battlefield sensors to have their batteries printed directly into them, making them rechargeable and also inexpensive and disposable.
In the short term, the MIT researchers are working to refine higher power and higher density batteries. This work involves examining new sets of materials, such as other types of metal phosphates, silicates and fluoride materials to develop new substances that are close to the theoretical maximum for density and power. The team also is seeking to develop materials that can be charged and discharged rapidly without any loss of power and to make them more stable. Another area that the MIT scientists are examining is developing materials that retain their properties after being stamped or poured.
Belcher’s research group has been working on using genetic engineering to control and synthesize inorganic materials for 10 years. The group’s first paper on the concept of selecting or evolving viruses for electronic materials appeared in Nature in 2000. As a graduate student, Belcher worked on Army and Navy funded research examining how natural structures such as abalone shells grow and how to apply this knowledge to developing self-forming nanostructures. Abalone shells are by mass 98 percent calcium carbonate and two percent protein. She explains that one of the properties of the shells is uniformity of the crystals at the nanoscale. This quality makes the material of the shell 3,000 times tougher than non-organically created materials. She adds that the shell’s substance is made at room temperature through self-assembly.
Using what she learned from her work with abalone shells, Belcher applied the proteins to other materials on the periodic table and discovered that they could control structures that were similar to calcium carbonate, but nothing else. “When I started my own lab, I began thinking about how do you get a protein that could control any protein that you want on the periodic table,” she says. Belcher then devised a method to test millions of proteins at one time to look for proteins that can grow and assemble inorganic materials.
Belcher’s researchers spent several years proving that proteins can be applied to viruses to form inorganic structures. The initial work used three to five semiconductors made from gallium arsenide and indium phosphide base materials. These proteins were used to bind the substances to the virus. However, she explains, the ultimate goal is to grow the materials through self- assembly. Her team became adept at manufacturing thin films and wires. This process was important because it meant that the scientists could make electronic components from inorganic materials. Once these processes were mastered, the team began to examine applications where the technology could make an immediate impact.
In the last three years, the MIT team began growing metals and metal oxides to form electrodes for battery anodes. Belcher notes that metal oxides appear in biological substances, which made them attractive for use in electrodes. In a paper that appeared in Science in 2006, her team described how it had engineered viruses to grow metal oxides such as cobalt oxide, which provides a higher specific electrical capacity than carbon- and graphite-based anodes. The goal of the effort was to develop new types of electrode materials.
This work led to the study of self-assembled battery electrodes and combining these substances with layered polymers. During this research, Belcher and her team began working on flexible, transparent, conformable batteries for use in clothing and handheld equipment.
Self-assembly of materials at the nanoscale depends on the application space, she says. For example, Belcher explains that it would be very difficult for her process to produce a microchip based on current designs. The MIT team examined a variety of options where self-assembly was an important factor, but where the individual components of a device did not have to be in an exact location. The self-assembly process creates a mesh of nanowires that are very effective at conducting electricity without requiring the individual wires to be in specific places.
Citing the example of her work with abalone shells, Belcher notes that the thickness of the material and the type of material are very important, but the exact location of each individual nanoscale tablet that make up the shell is random. “It’s that random offset of the tablets that has them form a brick wall structure and not a set of columns. It they were a set of columns, they wouldn’t be as tough as these laterally offset structures,” she says. Even in natural materials such as abalone shells, there is a level of self-assembly and organization, and then there is a level of randomness that helps the overall structure, she observes.
The MIT team considers its structures self-assembled because once the DNA sequence is present, it grows the virus that makes the inorganic material and then the resulting structure wires itself to the current collector. However, she notes that currently, researchers cannot dictate how the individual structures are arranged, although this may be possible in the future. The team is focusing on structural and material matters and in this process, the material’s relative proximity to the current collector is more important because these can be made more quickly and potentially impact new technologies faster, she says.
WEB RESOURCES
Massachusetts Institute of Technology, Department of Materials Science and Engineering: http://www-dmse.mit.edu




Comments