Tiny Machines Coalesce In the Spotlight
 |
| The porphyrin nanotubes developed by Sandia National Laboratory researchers John Shelnutt (l) and Zhongchun Wang use light to assemble themselves. These structures have a variety of potential applications in electronics, fuel cells and optics. |
Researchers have discovered a class of nanoscale devices that can self-assemble when exposed to light. These sub-microscopic structures may provide new methods for manufacturing electronic components such as photonic devices and memory storage systems for computers. Another potential application for the technology is in splitting water molecules to generate hydrogen for use as fuel.
Developed at Sandia National Laboratories,
Porphyrins are a class of light-sensitive molecules related to chlorophyll. In nature, they form part of the photosynthetic proteins and light-harvesting nanostructures found in plants and photosynthetic bacteria, John Shelnutt, leader of the Sandia research team, explains. These molecules also are active in many other proteins. Blood derives its red color from the porphyrins found in hemoglobin, for example.
The light-activated nanotubes can be made with small deposits of metals such as platinum and gold outside or inside the tube. The type of metal and its location on the nanotube can be chosen to determine the device’s function. For example, nanotubes with gold inside and platinum on the exterior, when coupled to a small band-gap semiconductor nanoparticle or film, may be able to split water into oxygen and hydrogen. The water-splitting process is driven by exposure to light, creating potential applications such as using solar power to generate hydrogen as a fuel source, Shelnutt speculates.
Nanotubes constructed from porphyrin are made of oppositely charged molecules that self-assemble in water at room temperature. This construction process differs from carbon nanotubes, which must be produced under high temperature and pressure. Although carbon nanotubes form very strong structures, Shelnutt asserts that porphyrin tubes possess a greater range of electronic and optical properties. He adds that scientists often attach porphyrins to carbon nanotubes to increase their utility.
A major advantage of porphyrin is its ability to form nanostructures with only the base materials and light. For example, manufacturing porphyrin nanotubes with metal components requires only water, platinum ions, ascorbic acid, and light from a projector lamp. Once combined, the metal components are grown within 30 minutes into nanostructures.
Shelnutt notes that the best organic conductors, especially those capable of forming into linear structures such as tubes or fibers, are related to porphyrins. “There is a good chance that they will be conductors or photoconductors, but we haven’t really started to look carefully at those properties yet,” he says.
The nanotubes grew out of research to remove heavy metals from water for waste remediation. This initial research effort examined the effectiveness of using porphyrins as photocatalysts to turn toxic metal ions into metal particles in water, reducing the metal’s ability to bond with other atoms to form potentially harmful molecules. “The idea was to see if we could pattern the porphyrins in some way to use them as little pumps that would generate metal in the vicinity of the porphyrin molecules and hopefully precipitate it [metals] there,” Shelnutt says.
The Sandia research team discovered the nanotubes when it began experiments to create nanoscale porphyrin structures. “There were some reports in the literature of porphyrin stripes appearing on surfaces. We were looking for better ways to do that, and they [the nanotubes] just cropped up by serendipity,” he explains.
But Shelnutt cautions that the research is still in an early stage and that little is known about the properties of porphyrin nanostructures. “We have seen interesting properties that we can use to build things, and we want to continue trying to build devices from them, but we also want to try to understand more about what’s causing some of the things that we’ve seen,” he says.
 |
 |
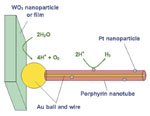 |
| One potential application for Sandia’s porphyrin nanotubes (top panel) is generating hydrogen by splitting water molecules. Nanotubes with gold wires in their center and platinum particles on the exterior form a gold ball at one end of the tube (middle panel). By using a tungsten oxide nanoparticle or film attached to the gold ball, the nanotube could become a water-splitting device (bottom panel). The gold nanowire and ball conduct electrons between the oxygen- and hydrogen-producing components of the device. Researchers already have demonstrated that nanotubes with platinum particles can produce hydrogen when exposed to light. According to Shelnutt, the device could use the entire visible and ultraviolet parts of the solar spectrum to produce hydrogen efficiently. |
This uninterrupted growth structure indicates that the tubes must have good electron conductivity through their walls to move electrons to the gold wire, but Shelnutt cautions that this is an unproven hypothesis. “If a reducing electron is generated in the wall of the tube at any place along the tube, why can’t it reduce gold right at that spot? That appears not to be what is happening. It appears to be able to travel to a place where gold growth is already initiated. It’s like a sink for electrons, and so they go to that site,” he says.
But building better structures and devices requires a deeper understanding of the growth process. To achieve this goal, work is proceeding to understand how these structures operate more efficiently than similar types of nanostructures, then to develop and test some of the proposed porphyrin devices.
Researchers are examining several potential electronics applications for the porphyrin nanotubes. One possible application is in generating hydrogen by splitting water molecules. Shelnutt notes that many other artificial photosynthesis systems using porphyrins also generate hydrogen from water. The difficulty lies in generating oxygen while simultaneously using water oxidation to split water molecules to make hydrogen at the tube’s platinum catalysts. Shelnutt’s group is considering coupling tungsten oxide, or tungsta, an inorganic photocatalyst, to the gold wire in the nanotubes. Tungsta uses blue and ultraviolet light to generate oxygen from water. Electrons generated in the tungsta particles run through the gold wire, replacing the electron donor molecules that they have used in generating hydrogen with the platinized nanotubes.
Shelnutt explains that this process allows both oxygen and hydrogen to be generated with water serving as the oxidant and reductant, eliminating the need for an additive as an electron donor. “If you want to make hydrogen, you have to make oxygen at the same time. Otherwise you have to make a lot of some other type of material that you probably don’t want,” he says.
One objective is to determine the feasibility of using water-splitting nanodevices to generate hydrogen. But cost remains a major consideration before the process can produce industrial quantities of hydrogen because the nanotubes use expensive metals such as gold and platinum. For example, if the tube’s conductivity is very high, then only a few platinum particles may be needed on each tube for the devices to function. However, Shelnutt observes that because platinum already is used in fuel cell construction, such large-scale use of the rare metal may create competition between fuel cell manufacturers and power generation industries.
The technology also has potential electronics applications such as memory storage and photonic devices. Shelnutt speculates that it may one day be possible to use one porphyrin molecule to store a positive or negative electrical charge as a memory device. “But for now, if you could control the growth of these tubes so that you could put them in arrays with uniform tube length, you might think of this as a self-organized system for creating memory cells,” he says.
Other uses include photonic and optical systems and sensors. Besides round tubes, Sandia researchers have made a variety of other nanostructures such as tubes with rectangular cross-sections and structures resembling planks and fibers. Shelnutt notes that some of these have interesting optical properties such as resonance light scattering, but he cautions that little is known about their properties. “We’ve seen interesting things, and we’re pursuing those, but it’s going to be a while before we know how useful they are,” he says.
Web Resource
Sandia National Laboratories: www.sandia.gov




Comments